when winter comes, psychosis about the spirit of Christmas, presents and the incredibly hard decision making of where to spend it begin. At that moment snow is just a detail, because of the slush we wade in, trying to reach the bus station, or only the necessary background for the feast. Have you ever thought about what is it made of, what a wonderful creation is the snowflake and what amazing kinds of snowflakes there are?
THE BIRTH
 The story of every single snowflake begins with the vapor of water from the oceans, lakes and rivers.
The story of every single snowflake begins with the vapor of water from the oceans, lakes and rivers.When air is cooled up, there comes a specific moment when vapour begins to condense. When it is happening near the ground, water may condense as dew over the plants. High above the ground, water vapour condenses on dust particles flying in the air. It condenses as drops, immeasurably small, every one with, at least, one dust particle in it. Clouds are aggregates of huge amount of such miniature drops.
In winter, snow clouds are formed mainly by liquid water drops, even when temperature is under the level of freezing. Water is overcooled. As clouds are cooling up further, drops begin to freeze. The process begins at about -10 C, but it is gradual and not all the drops freeze simultaneously.
When freezing, every drop becomes ice, surrounded by other drops in the cloud, which are still not frozen. Ice is growing as more and more vapour condenses on its surface, forming snowflake. With the growth of ice other drops of water slowly evaporate and saturate the air with more and more vapour.
MORPHOLOGY
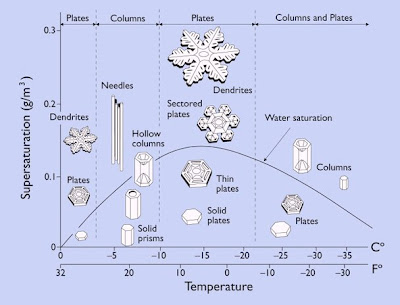
When scientists began to grow snow crystals in lab conditions, they discovered that their shape depends on temperature and saturation. This behavior is shown on the diagram below.
Crystals form more simple shapes when humidity is lower. The most complicated shapes are formed in extremely high humidity.
 Why shapes are so dependable on temperature and so different, it is still a mystery. It is clear that the growth depends on the exact quantity of vapour molecules, integrated in the growing ice crystal, but most of the physical processes are still not well revealed.
Why shapes are so dependable on temperature and so different, it is still a mystery. It is clear that the growth depends on the exact quantity of vapour molecules, integrated in the growing ice crystal, but most of the physical processes are still not well revealed.  So, next time when the snow touches you, stretch your hand and shelter several melting crystals on your glove. Look them closely with the eyes of someone who knows something about them. Make a wish for the new year and feel happy that Nature had touched you with one of its wonderful creations.
So, next time when the snow touches you, stretch your hand and shelter several melting crystals on your glove. Look them closely with the eyes of someone who knows something about them. Make a wish for the new year and feel happy that Nature had touched you with one of its wonderful creations.
This article is based on facts from SnowCrystals.com.
Photographs: SnowCrystals.com